Introduction
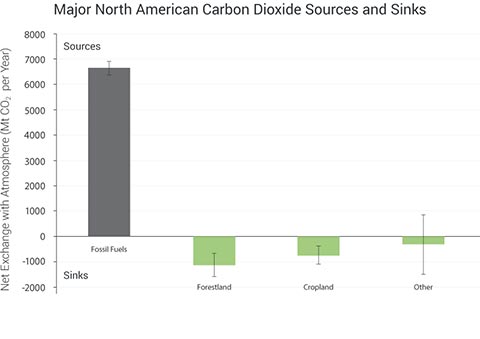
Biogeochemical cycles involve the fluxes of chemical elements among different parts of the Earth: from living to non-living, from atmosphere to land to sea, and from soils to plants. They are called “cycles” because matter is always conserved and because elements move to and from major pools via a variety of two-way fluxes, although some elements are stored in locations or in forms that are differentially accessible to living things. Human activities have mobilized Earth elements and accelerated their cycles – for example, more than doubling the amount of reactive nitrogen that has been added to the biosphere since pre-industrial times.6,24 Reactive nitrogen is any nitrogen compound that is biologically, chemically, or radiatively active, like nitrous oxide and ammonia, but not nitrogen gas (N2). Global-scale alterations of biogeochemical cycles are occurring, from human activities both in the U.S. and elsewhere, with impacts and implications now and into the future. Global carbon dioxide emissions are the most significant driver of human-caused climate change. But human-accelerated cycles of other elements, especially nitrogen, phosphorus, and sulfur, also influence climate. These elements can affect climate directly or act as indirect factors that alter the carbon cycle, amplifying or reducing the impacts of climate change.
Climate change is having, and will continue to have, impacts on biogeochemical cycles, which will alter future impacts on climate and affect our capacity to cope with coupled changes in climate, biogeochemistry, and other factors.